Determination of chromatographic content of dapsone tablets (Dapsone) using the ACQUITY UPC 2 system
purpose
Using Waters (Waters®) ACQUITY UPC 2 ™ system converts Pharmacopoeia dapsone content of normal phase HPLC method for measuring a supercritical fluid chromatography (SFC) method.
background
Currently, the United States Pharmacopoeia (USP) specifies a normal phase HPLC method for the analysis of tablets containing dapsone (4,4'-diaminodiphenyl sulfone, CAS #80-08-0 ). The isocratic separation was carried out using a 4.0 x 300 mm, 10 μm silica gel column (L3), and the mobile phase was a mixed solution of n-hexane, isopropanol, acetonitrile and ethyl acetate (7:1:1:1). The run time of the method was approximately 12.5 min (twice the peak time of the last major peak, flow rate 1.5 mL/min). As in most Pharmacopoeia methods, this method is proven and reliable. However, this method uses a solvent of n-hexane and ethyl acetate. Many laboratories want to reduce the use of these solvents for health, safety and environmental reasons. Supercritical liquid chromatography (SFC) is a normal phase chromatographic separation technique that uses CO 2 as the main mobile phase and a polar solvent such as methanol as a modifier. Since the principle of SFC is similar to the principle of HPLC, the current method should be able to convert to SFC method, reduce solvent consumption and processing, reduce the cost per analysis, and enhance health, safety and environmental protection. Chromatographic methods converted to SFC must maintain data quality and must be obtained with current normal phase chromatography
The method is consistent with the experimental results.
The ACQUITY UPC 2 system is ideal for laboratories looking for a more efficient and cost-effective method for analyzing dapsone tablets , which enhances health, safety and environmental protection.
solution
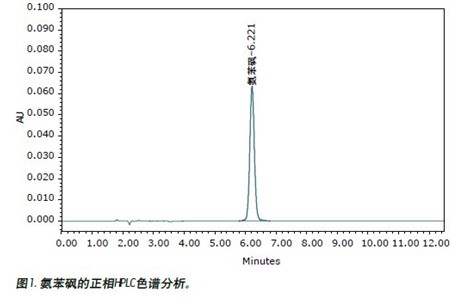
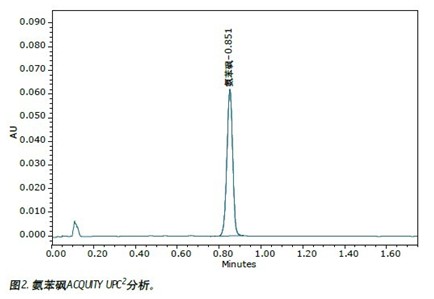
Samples of dapsone standards and tablets were prepared and analyzed using current USP (USP) methods, as shown in Figure 1 (this sample is also used for SFC analysis). The analysis results using the current USP method are compared with the results obtained using the ACQUITY UPC 2 method, as shown in FIG.
The conditions of the SFC method are as follows:
Column: ACQUITY UPC 2 BEH, 3.0 x 50 mm, 1.7 μm
Column temperature: 45 °C
Mobile phase: 85% CO2: 15% MeOH
Flow rate: 3.0 mL/min,
Back pressure: 130 bar / 1885 psi
Detector: UV / PDA, 254 nm
The adaptive conditions listed in the Pharmacopoeia method are minimum requirements (relative standard deviations must not exceed 2%). The standard product was repeatedly injected six times. The relative standard deviation (%) of the retention time and peak area obtained by the normal phase HPLC method was <0.1%, <1.1%, respectively. UltraPerformance Convergence ChromatographyTM (UPC 2 ) Ultrasound Convergence ChromatographyTM (UPC 2 ) The results of repeated 6 injections meet the USP Pharmacopoeia system compliance requirements (retention time RSD value 0.8%, peak area RSD value 0.9%), and running speed ( 1.75 min) greatly accelerated. The analytical results of the two methods for determining tablet samples were highly consistent. In this example, each normal phase HPLC analysis used 13.1 mL of n-hexane, 1.9 mL each of isopropanol, acetonitrile and ethyl acetate. In contrast, the UPC 2 method consumes only about 0.50 mL of methanol. This demonstrates that the use of organic solutions can be greatly reduced by converting the normal phase chromatography method to the UPC 2 method. Based on current solvent prices, the cost of each normal phase HPLC analysis is approximately $1.08; in contrast, UPC 2 is only $0.01.
to sum up
The AC Pharmacy HPLC method can be successfully converted to the UPC 2 method using ACQUITY UPC 2 . The data from this new UPC 2 method is comparable to, or even better than, current HPLC methods; the speed is seven times faster than current HPLC methods and consumes less solvent. By getting high-quality analytical data faster, labor productivity increases and the cost of analysis per sample decreases. The ACQUITY UPC 2 System is an ideal solution for the laboratory to convert the current normal phase HPLC method into a more efficient and cost-effective UPC 2 method, while also enhancing health, safety and environmental protection.
About Waters Corporation ()
For more than 50 years, Waters Corporation (NYSE: WAT) has made significant advances in medical services, environmental management, food safety and global water quality monitoring by providing practical and sustainable innovations that have created business for laboratory-related organizations. Advantage.
As a pioneer in a range of separation science, laboratory information management, mass spectrometry and thermal analysis technologies, Waters technology's breakthroughs and laboratory solutions create a lasting platform for customer success.
With a $1.85 billion revenue in 2011, Waters continues to lead customers around the world in exploring science and achieving excellence.
For more than 50 years, Waters Corporation (NYSE: WAT) has made significant advances in medical services, environmental management, food safety and global water quality monitoring by providing practical and sustainable innovations that have created business for laboratory-related organizations. Advantage.
As a pioneer in a range of separation science, laboratory information management, mass spectrometry and thermal analysis technologies, Waters technology's breakthroughs and laboratory solutions create a lasting platform for customer success.
With a $1.85 billion revenue in 2011, Waters continues to lead customers around the world in exploring science and achieving excellence.
# # #
Waters, UPC 2 , UltraPerformance Convergence Chromatography, ACQUITY and UPLC are registered trademarks of Waters Corporation.
Spindle Analyzer,Cnc Spindle Analysis,Spindle Analyzer For Machine Tool,Api Swivel Check
Automated Precision Inc. , https://www.apiasean.com