Model NO.: PP305
Pharmaceutical Technology: Chemical Synthesis
Drug Reg./Approval No.: 161003
Specification: 40mg/ml
Origin: China
Model NO.: PP305
Pharmaceutical Technology: Chemical Synthesis
Drug Reg./Approval No.: 161003
Specification: 40mg/ml
Origin: China
This product omeprazole sodium is mainly used in duodenal ulcer and cha-ai syndrome (gastrin) and can be used in gastric ulcer and reflux esophagitis.• name of foreign language
Omeprazole Sodium for Injection
• drug name
Sodium omeprazole sodium injection
• prescription drugs
Prescription drugs
• major applicability
Peptic ulcer bleeding
• adverse reactions
Nausea, diarrhea, abdominal pain, abnormal sensation, dizziness or headache
• drug types
The digestive system
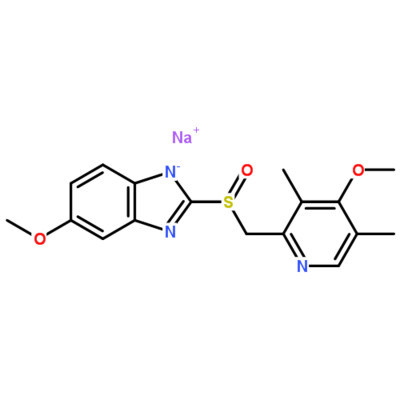
Â
Drug instructions
[drug type] digestive system
[Chinese name] omeprazole sodium injection
Sodium omeprazole sodium injection
[product English name] Omeprazole Sodium for Injection
The main function is: the peptic ulcer hemorrhage, anastomotic ulcer hemorrhage.Acute gastric mucosal injury caused by acute gastric mucosal injury and non-steroidal anti-inflammatory drugs in the stress state;It is also used to prevent the prevention of rehemorrhage after gastric surgery, such as brain hemorrhage, severe trauma, etc.General anesthesia or large hand surgery and weak coma patients to prevent acid reflux combined with aspiration pneumonia.
drug character this product is white loose piece or powder, special solvent is colorless transparent liquid.
[pharmacological action]
 This product is the stomach cells proton pump inhibitor, can specifically inhibit the top of the wall cell membrane of secretory microtubules and intracytoplasmic tubular bubble of H +, K + atpase, effectively inhibit the secretion of stomach acid.Because H +, K+- atpase is the last process of wall cell lacic acid, this product has strong acid suppression ability.
[drug interaction]
 (1) of this product can prolong diazepam, phenytoin sodium and other liver the efficacy of oxygen metabolism of drugs, such as the product and phenytoin sodium share, need to carefully monitor disease, and should take into consideration the phenytoin sodium reduction.
(2) there may be interaction with drugs that are metabolized by cytochrome P450 enzyme system (such as warfarin).
Adverse reactions can be seen with mild nausea, diarrhea, abdominal pain, abnormal sensation, dizziness or headache, etc., without affecting the treatment.
[product specification] 40mg (according to omeprazole)
[pharmaceutical ingredients] omeprazole sodium
Although pregnant women medication this product are not found in animal experiments has adverse effects to the gestation and lactation, or toxic or teratogenic effects on the fetus, but suggested that pregnancy and lactation women as far as possible need not.
Children's use of this product has limited experience.
Elderly patients need not adjust the dosage.
[usage] intravenous injection.One 40mg, 1~2 times per day.The 10ml special solvent is injected into the small bottle of freeze-dried powder, and it is forbidden to be dissolved in other solvents.The product must be used within 2 hours after dissolving and the time is not less than 20 minutes.
The storage method is sealed and kept in a cool dark place.
considerations
(1) this product can inhibit the secretion of stomach acid and has a long time, so it is not suitable to take other antacids or acid inhibitors when applying this product.In order to prevent the excessive acid suppression, in general peptic ulcer and other diseases, it is not recommended that large dosage of long-term application (zhuo - ai syndrome is an exception).
(2) because this product can significantly increase the pH of the stomach, it may affect the absorption of many drugs.
(3) patients with impaired renal function must not adjust the dosage;Patients with impaired liver function need to be reduced as appropriate.
(4) when treating gastric ulcer, it is necessary to eliminate gastric cancer before using this product, so as not to delay the diagnosis and treatment.(5) the animal experiment, after long-term use of this product, the observed high stomach secrete hormone levels, and secondary ECL - cell increased, and the occurrence of benign tumors, the change in other acid suppression and gastric resection most exposed.
 This product omeprazole sodium is mainly used in duodenal ulcer and cha-ai syndrome (gastrin) and can be used in gastric ulcer and reflux esophagitis.
• name of foreign language
Omeprazole Sodium for Injection
• drug name
Sodium omeprazole sodium injection
• prescription drugs
Prescription drugs
• major applicability
Peptic ulcer bleeding
• adverse reactions
Nausea, diarrhea, abdominal pain, abnormal sensation, dizziness or headache
• drug types
The digestive system

Â
Drug instructions
[drug type] digestive system
[Chinese name] omeprazole sodium injection
Sodium omeprazole sodium injection
[product English name] Omeprazole Sodium for Injection
The main function is: the peptic ulcer hemorrhage, anastomotic ulcer hemorrhage.Acute gastric mucosal injury caused by acute gastric mucosal injury and non-steroidal anti-inflammatory drugs in the stress state;It is also used to prevent the prevention of rehemorrhage after gastric surgery, such as brain hemorrhage, severe trauma, etc.General anesthesia or large hand surgery and weak coma patients to prevent acid reflux combined with aspiration pneumonia.
drug character this product is white loose piece or powder, special solvent is colorless transparent liquid.
[pharmacological action]
 This product is the stomach cells proton pump inhibitor, can specifically inhibit the top of the wall cell membrane of secretory microtubules and intracytoplasmic tubular bubble of H +, K + atpase, effectively inhibit the secretion of stomach acid.Because H +, K+- atpase is the last process of wall cell lacic acid, this product has strong acid suppression ability.
[drug interaction]
 (1) of this product can prolong diazepam, phenytoin sodium and other liver the efficacy of oxygen metabolism of drugs, such as the product and phenytoin sodium share, need to carefully monitor disease, and should take into consideration the phenytoin sodium reduction.
(2) there may be interaction with drugs that are metabolized by cytochrome P450 enzyme system (such as warfarin).
Adverse reactions can be seen with mild nausea, diarrhea, abdominal pain, abnormal sensation, dizziness or headache, etc., without affecting the treatment.
[product specification] 40mg (according to omeprazole)
[pharmaceutical ingredients] omeprazole sodium
Although pregnant women medication this product are not found in animal experiments has adverse effects to the gestation and lactation, or toxic or teratogenic effects on the fetus, but suggested that pregnancy and lactation women as far as possible need not.
Children's use of this product has limited experience.
Elderly patients need not adjust the dosage.
[usage] intravenous injection.One 40mg, 1~2 times per day.The 10ml special solvent is injected into the small bottle of freeze-dried powder, and it is forbidden to be dissolved in other solvents.The product must be used within 2 hours after dissolving and the time is not less than 20 minutes.
The storage method is sealed and kept in a cool dark place.
considerations
(1) this product can inhibit the secretion of stomach acid and has a long time, so it is not suitable to take other antacids or acid inhibitors when applying this product.In order to prevent the excessive acid suppression, in general peptic ulcer and other diseases, it is not recommended that large dosage of long-term application (zhuo - ai syndrome is an exception).
(2) because this product can significantly increase the pH of the stomach, it may affect the absorption of many drugs.
(3) patients with impaired renal function must not adjust the dosage;Patients with impaired liver function need to be reduced as appropriate.
(4) when treating gastric ulcer, it is necessary to eliminate gastric cancer before using this product, so as not to delay the diagnosis and treatment.(5) the animal experiment, after long-term use of this product, the observed high stomach secrete hormone levels, and secondary ECL - cell increased, and the occurrence of benign tumors, the change in other acid suppression and gastric resection most exposed.
Â
Casing Safety Clamp,Safety Clamps,Drill Collar Clamps
Kaihao Petroleum Equipment Co., Ltd. , http://www.dypetroleumequipment.com