The FDA approved the Philips heart sensor S4-1, which is used in the company's diagnostic ultrasound device Lumify. New add-on features bring cardiac imaging into the field of portable diagnostics, and have now allowed doctors to scan other parts of the gallbladder and lungs.
The Lumify suite launched a year ago includes an app and two sensors for Android smartphones, as well as online access and Philips technical support. The app connects to the cloud and clinicians can access data and images via Philips' HealthSuite digital platform. In addition to heart and abdomen scans, Lumify also offers obstetric/gynaecological examinations, and FAST (ultrasound as a focus on trauma assessment).
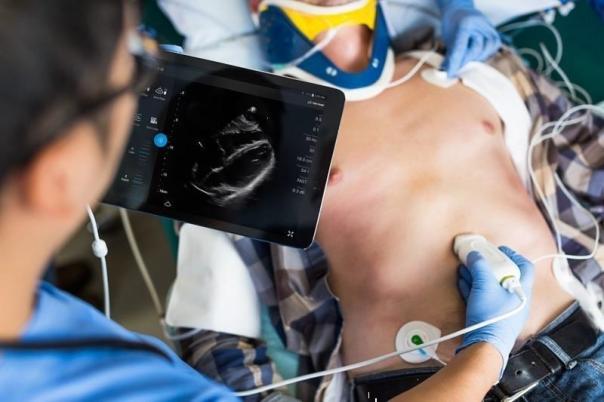
Philips Diagnostic Ultrasound Smart Device Lumify
China Extract Powder For Use As Dietary Supplement Extract Powder, Extract Powder Manufacturer
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.anabolicsteriod.com