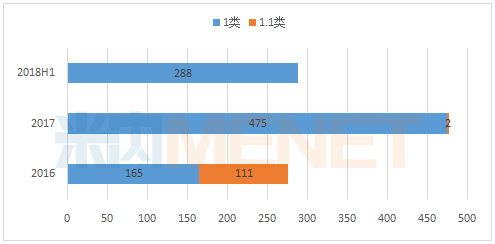
Key indicators Medical Network July 23 hearing of drug development is a manifestation of corporate innovation medicine, it is one of the focus of the industry. With the promotion of innovation policies, the enthusiasm for the development of new drugs in pharmaceutical companies is growing. As of June 30, 2018, CDE had 288 applications for Class 1 new drugs, which exceeded half of the applications in 2017, and exceeded the number of applications for the whole year of 2016. Under the influence of the accelerated review and approval, part of the The application for entering the CDE for half a year has been completed and approved.
The first half of the first class of new drug contracting situation
Under the blessing of various encouraging policies, the application for Class 1 new drugs is increasing year by year in recent years. In the first half of 2018, CDE has applied for 288 new types of new drugs, which has exceeded half of the applications in 2017 and exceeded the full year of 2016. The number of applications.
In the first half of 2016-2018, the application for CDE1 new drug application (according to the acceptance number)
(Source: Mene Net MED Drug Evaluation Database 2.0, the same below)
CD19 is a hot target in the first half of the year
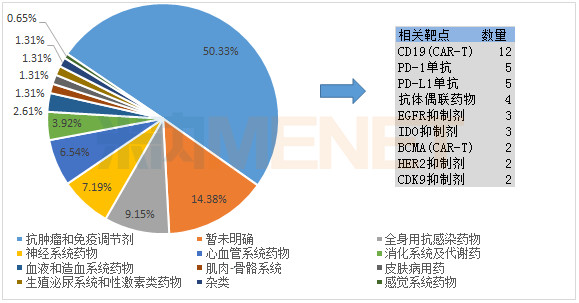
From the field of treatment, antitumor drugs is still a hotspot drug registration class declaration. Since the first CAR-T therapy entered the CDE at the end of 2017, CAR-T therapy has emerged in the anti-tumor field in the first half of 2018, with CD19 becoming a hot target.
The proportion of each treatment category of new drugs in the first half of 2018 (according to the number of common names of drugs)
Henry half declare a class of drugs 6
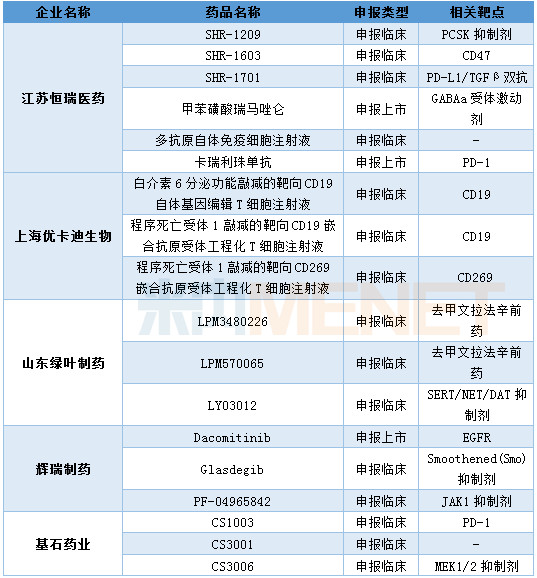
There are 5 companies with more than 3 types of new drugs in the first class. Jiangsu Hengrui Medicine has reported 6 Class 1 new drugs in the first half of the year, covering the fields of anti-tumor, nervous system and cardiovascular system. Shanghai Youkadi Bio has three CART therapies, the main targets being CD19 and CD269.
Enterprises with more than 3 new drugs declared in the first half of 2018 (according to the number of common names of drugs)
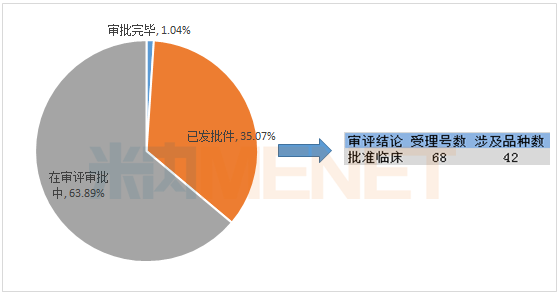
42 drugs have been approved for clinical use
As of the date of statistics, in the first-half of 2018, CDE had 35.07% of the acceptance number in the first-half of 2018, and the status of the acceptance number was “issuedâ€. In the application for the status of “issued documentsâ€, 68 applications were accepted. The number was approved for clinical use and involved 42 drugs .
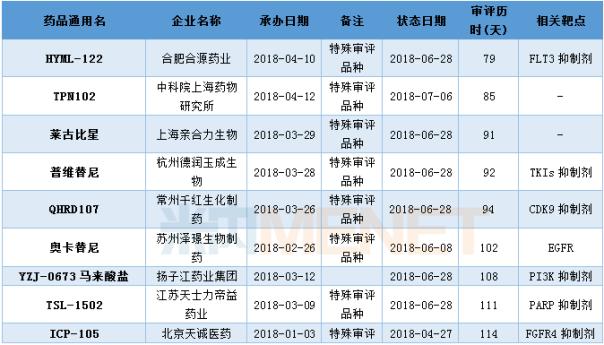
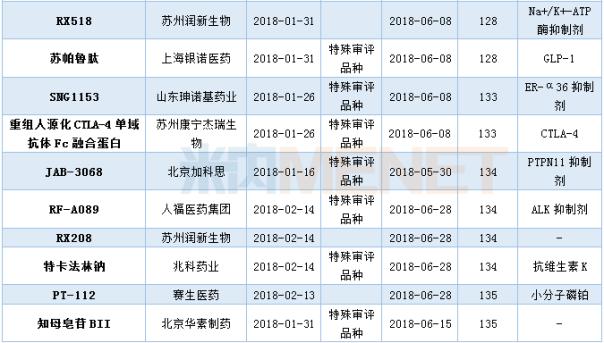
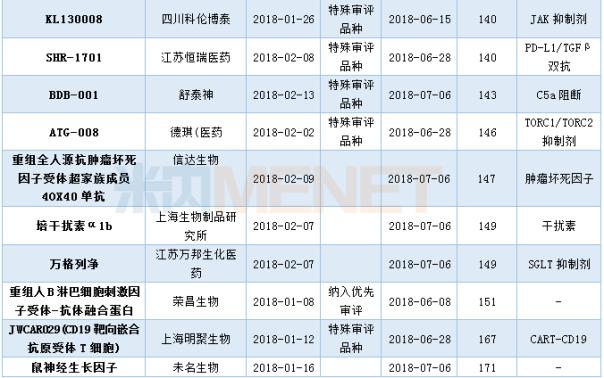
Approval of Class 1 New Drugs Hosted in the First Half of 2018
(Data statistics as of July 10, 2018, the same below)
HYML-122 has the fastest review
With the increase of the speed of examination and approval, the review and approval time for the registration of new drug registrations is gradually shortening. In the first half of 2018, many varieties of new drugs under the contract of CDE have been approved, including Hefei Heyuan Pharmaceutical. The HYML-122 review was the fastest, and it took only 79 days to get the clinical approval from the CDE to the SFDA. (If there are errors or omissions in related drug targets, please correct me)
In the first half of 2018 , CDE sponsored a class 1 new drug approved clinical situation

Oat Powder is produced by the specific enzymatic hydrolysis and spray drying technology, so it is called Hydrolyzed oat powder or Enzymolysis Oat Powder. It has a pleasant oat fragrance with creamy mouthfeel and smooth texture. It retains all the soluble nutrients of oats, such as high-quality plant protein, fat, soluble dietary fiber(3-glucan>3%). It is high-viscosity and slightly sweet, easy to digest and absorb. It is stable and good resistance to acid and heat treatment after dissolution in water.
Oat Powder,Oat Milk Powder,Hydrolyzed Oat Flour,Hydrolyzed Oat Powder,Enzymolysis Oat Powder
Xi'an Gawen Biotechnology Co., Ltd , https://www.amulyn-bio.com