Release date: 2016-06-16
On June 14, 2016, the US Food and Drug Administration (FDA) approved a new obesity treatment device that uses surgically placed tubing to draw a portion of the stomach contents after each meal.
The AspireAssist device should not be used in patients with eating disorders, and it is not intended for short-term use in patients who are moderately overweight. It is intended to help obese patients ages 22 and older have weight loss, 35 to 55, and patients who cannot achieve and maintain weight loss through non-surgical weight loss treatment.
William Maisel, MD, MPH, deputy director of science and chief scientist at the FDA's Center for Devices and Radiation Health, said: "The AspireAssist method helps improve effective heat absorption, which is a key principle in weight treatment." "Patients need to be treated by their health care." Providers regularly monitor and follow up on lifestyle programs to help them develop healthy eating habits and reduce calorie intake."
To place the device, the surgeon inserts a tube into the stomach with an endoscope through a small incision in the abdomen. There is a disc-type port valve on the outside of the body that flushes the skin of the abdomen and is attached to the tube and held in place. About 20 to 30 minutes after the meal, the patient attaches the device external connector and tubing to the port valve, opens the valve and draws the contents. Once opened, the food material is pumped through the tubing and into the toilet for about 5 to 10 minutes. The device takes about 30% of the calories burned.
FDA review results from a clinical trial 111 patients treated with AspireAssist and appropriate lifestyle treatment, and 60 control patients received only lifestyle treatment. One year later, the average weight loss of AspireAssist patients was 12.1% of their overall weight compared with 3.6% of the control patients.
Clinical trial results also suggest that both patient groups have small improvements often associated with obesity, such as diabetes, hypertension, and quality of life. These improvements may be attributed to lifestyle treatments, including nutrition and exercise counseling.
Patients need to be frequently monitored by health care providers to shorten the tube as they lose weight and abdominal circumference, so the discs remain flushed to their skin. Frequent medical visits also require monitoring device usage and weight loss and providing counseling for lifestyle treatments. The device also has a safety feature that tracks the number of connections to the port and automatically stops after 115 cycles (approximately 5 to 6 weeks of treatment); the patient must return to a medical visit to continue treatment of the device replacement parts obtained. This safety feature helps ensure that the patient is properly used during treatment.
Side effects associated with AspireAssist use include occasional indigestion, nausea, vomiting, constipation and diarrhea.
Endoscopic surgery placement of the stomach tube is associated with risks including sore throat, pain, bloating, indigestion, bleeding, infection, nausea, vomiting, sedation-related breathing problems, inflammation of the abdominal lining, medial ulcer of the stomach, pneumonia, stomach or The small intestine wall was accidentally punctured and died.
Risks associated with port valve abdomen opening include abdominal discomfort or pain, skin irritation around the placement site, hardening or inflammation, leakage, bleeding and/or infection around the placement site and movement of the device into the stomach wall. All have the necessary removal device. After removal of the device, there may be a risk of persistent fistula, an abnormal passage between the stomach and the abdominal wall.
There are certain conditions in which patients are contraindicated with AspireAssist, including uncontrolled high blood pressure, diagnosed bulimia, diagnosed binge eating disorder, nocturnal eating syndrome, certain types of previous abdominal surgery, pregnancy or breastfeeding, inflammatory bowel Pathological disease or stomach ulcers. AspireAssist is also contraindicated in patients with severe pulmonary fire cardiovascular disease, coagulopathy, chronic abdominal pain, or high-risk patients with medical complications from an endoscopic procedure.
The AspireAssist system is manufactured by Aspire Bariatrics, located in King Prussia, Pennsylvania.
Source: Tangzhongming's blog
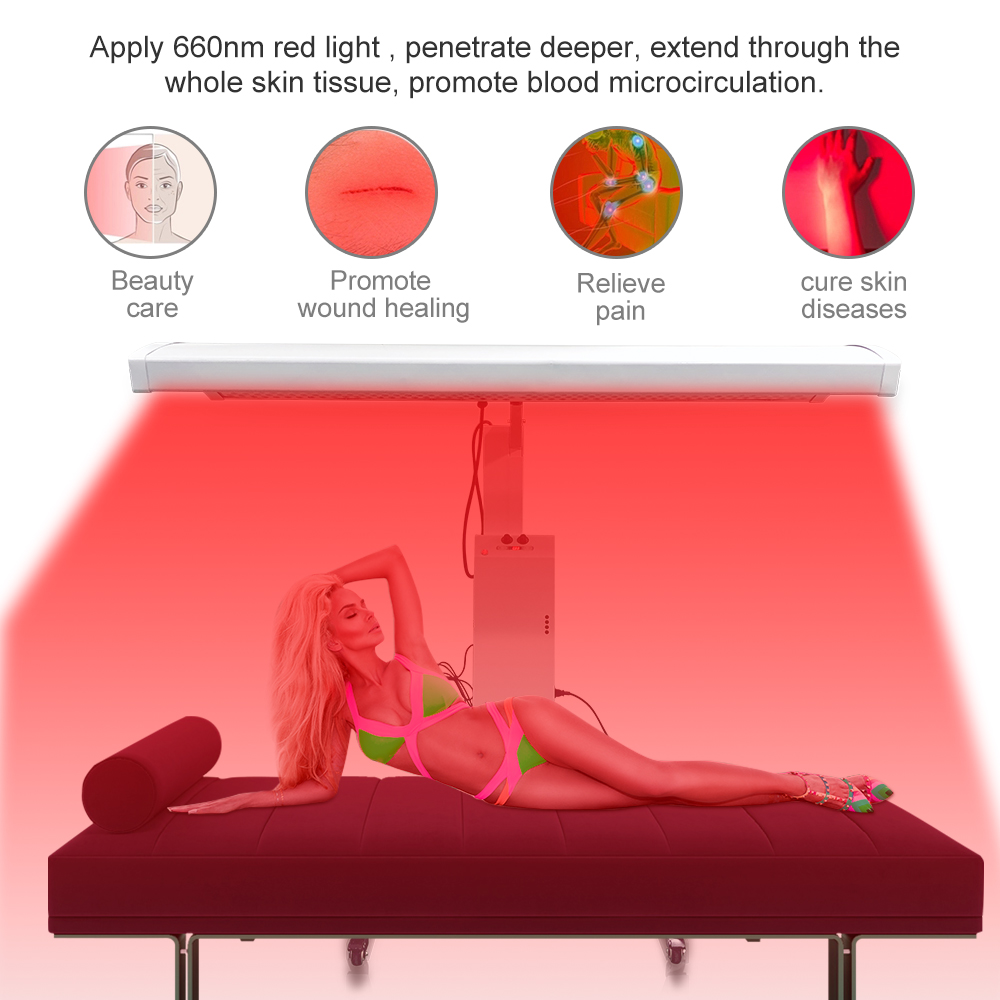
Full Body Red Light Therapy Panel Infrared Light Device Red Light Therapy Panel
Red Light Therapy Panel,red light panel,pdt machine,red light therapy full body,Infrared Light Therapy,Infrared device
Red LED light therapy equipment for plastic surgeons, dermatologists, estheticians, and other licensed skincare and wellness professionals.
Red light therapy has been studied for its potential to improve skin health, reduce inflammation, enhance muscle recovery, and promote overall well-being. It is often used in professional settings, such as spas and wellness centers, but there are also portable at-home versions available for personal use.
Red light therapy panels can be used by positioning them close to the body or by standing in front of them. The treatment duration and frequency vary depending on the desired outcome and the specific device being used. It is generally considered a safe and non-invasive therapy with minimal side effects.
It's important to note that while red light therapy has shown promising results in some studies, more research is needed to fully understand its effectiveness and potential applications. As with any medical or wellness treatment, it is advisable to consult with a healthcare professional before starting red light therapy.
Red Light Therapy Panel,red light panel,pdt machine,red light therapy full body,Infrared Light Therapy,Infrared device
Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.szlighttherapymachine.com